Post-release monitoring
Introduction
Several studies have shown that non-target species are attacked in the field by introduced biological control agents. However, Hopper (1998) pointed out that few studies demonstrated that such attack had any impact on population density of a non-target species.
Stanley and Julien (1998) noted that too few biological control programmes continued their efforts to the point where pre-release predictions were validated by post-release studies of biological control agents. Also Holt and Hochberg (2001) concluded from theoretical studies that attack rates alone do not adequately describe risk to non-target species.
A literature review for biological control of insects showed that data on post-release impacts were reported for less than 2% (of over 5000) of classical biological control introductions (Lynch et al. 2001). Extrapolating from their data, they estimated that just under 10% of instances of non-target attack may have led to population impacts, and hence over 600 non-target insect species may have been affected at the population level throughout the history of biological control. The authors of the review suggested that there exists major under-reporting of adverse effects of biological control agent introductions.
For weed biological control, the safety record appears to be very high, with only two of over 300 species that have been released worldwide affecting non-target species at the population level. Post-release monitoring is not routine in weed biological control programmes, and so under-reporting of non-target effects may exist; however, herbivores and their impact on plants are easier to assess than effects of parasitoids, invertebrate predators or entomopathogens.
Desirability of monitoring
The EPA does not normally apply controls to release approvals requiring post-release monitoring because controls are intended to reduce risk. However, the EPA encourages post-release monitoring because the data would help validate decisions, and hence inform future decisions on biological control agents. Furthermore the EPA does have a requirement under Section 148b to monitor effectiveness of HSNO on reducing impacts.
Funding for monitoring
Post-release monitoring is not normally used as a condition of approval for
biological control agents, mainly because it does not manage risk, since the
release has already been made. Furthermore, the EPA is aware that
monitoring depends upon adequate funding that cannot always be guaranteed.
However, some post-release monitoring research, and retrospective studies of
pervious decisions is being carried out in the research programme
Better Border Biosecurity (B3) [http://www.b3nz.org]
in a sub-programme "Improved Biosafety for EPA Decisions".
This work is funded by a research collaboration made up of AgResearch, Plant and
Food Research, Scion, Landcare Research, and also Lincoln University.
Other sources of funding for work like this could come from the
beneficiaries of the biological control programme, who perhaps want to monitor
post-release impacts on the target pest, and adding non-target species to this
work can sometimes be quite cost-effective.
![]()
Monitoring methods
Barratt et al. (2006) recently reviewed post-release monitoring research for biological control agents and provided guidelines. The guidelines are based on previously published information and case studies detailed in the same chapter. In practical terms, a logical method of evaluating non-target effects is to combine this with measuring target effects using similar methods as far as possible. This can be appropriate when target and potential non-target hosts are in the same environment: for example, in early post-release studies and in cases where the biological control agent is very immobile; however, impacts often extend beyond the target host environment, requiring a different approach.
These guidelines for post-release monitoring are taken from
Barratt et al. (2006), and
reproduced here with permission from CABI, Wallingford, UK.
![]()
Direct effects on non-target, beneficial or valued species
In preparation for post-release studies, pre-release opportunities can be taken to enhance robust post-release investigations:
- Population monitoring of a range of potential 'high risk' non-target species
to give a baseline for post-release evaluation. The species list can come from:
- knowledge of host range in country of origin;
- quarantine host range studies in country of proposed new release which indicate potential non-target hosts (van Lenteren et al. 2006);
- literature on known non-target hosts from releases elsewhere;
- knowledge of phylogenetic and ecological affinities of target with fauna in country of proposed release;
- known beneficial species in proposed country of release;
- Surveys of fauna and ecosystem processes in proposed country of release.
- Life table analysis for one or two 'high risk' non-target species so that post release impacts can be quantified. Candidate species can be selected from quarantine data where potential non-target effects have been determined.
- Surveys of potential non-target species in the target host environment.
- Surveys to determine if and where the target pest occurs, outside of the environment within which it is known as a pest, can indicate potential environments in which non-target effects could occur.
- Similarly, surveys outside of the target host environment can be useful for collecting information on the range and distribution of native hosts phylogenetically
- related to the target host.
- For some biological control agents, phylogenetic 'relatedness' to potential nontarget species is less important than habitat, e.g., some leaf-miner parasitoids are able successfully to attack leaf miners from a number of insect orders, but might show specificity to the host plant of the leaf miner complex.
- Information on the mobility of the proposed biological control agent and the target host are useful. Even if the biological control agent is relatively immobile, a host capable of wide dispersal can potentially carry the agent to new habitats.
Then post-release
- Determine which, if any, non-target species (including beneficials and other pest species where appropriate) are attacked in the field by sampling in the target pest habitat and beyond; determine which species and the proportion of non-target populations being attacked.
- Field evaluation of non-target attack should incorporate appropriate spatial, temporal and seasonal scales.
- For predators, gut analysis methods can be used to determine diet breadth e.g., Hoogendorn and Heimpel (2003).
- For herbivorous control agents, long-term monitoring using standardized techniques plus regular observations.
- Development of food web models using stable isotope ratios may be helpful in quantifying how invasions and biological control introductions at various trophic levels affect resource flows in different habitats.
If non-target impact is identified in the field
- Regular sampling programme is useful for determining comparative phenology of the target host and one or more identified non-target species to help predict impact.
- Once a biological control agent is established in a non-target population, life-table analysis is ideal for estimating if impact is feasible. The dynamics here are likely to change over time and space. In weed-control programmes, long-term monitoring of target and non-target populations is essential. Attack alone does not imply that a population level effect will materialize, but declines or increases in plant populations should be recorded.
- Consider developing and testing a predictive model of population impact.
- Match pre-release predictions with post release evaluation. If they match up poorly, ascertaining reasons for this is of value to practitioners and regulators for future biological control proposals.
- If a beneficial species is attacked, it might be necessary to determine how this affects the benefits for which the species is valued.
- Inform the EPA of your findings.
If non-target impact is not identified in the field
- Maintain a low-intensity monitoring programme if possible, e.g., annual sampling at a small number of key sites near such release sites, or at areas of high target impact, and presumably of high biological control agent activity. If a biological control agent is very effective in reducing target populations, there may be a period when the agent is under pressure to locate suitable alternative hosts.
- Competition with or displacement of other natural enemies
- Pre-release information on existing natural enemies (parasitoids, pathogens, predators and herbivores) of the target host, and particularly on identified potential non-target hosts, is useful, so that indirect effects can be ascertained post-release.
- Post-release, non-target hosts can be sampled over time to determine the extent of displacement of natural enemies by the newly released biological control agent and these results compared with pre-release data. This can be integrated with the investigations of direct non-target effects, above.
Longer term impacts
There have been a small number of studies carried out to determine the longer-term impacts of introduced biological control agents but few have considered impacts in natural environments. A study was carried out in New Zealand to look at impacts of the parasitoid Microctonus aethiopoides, a biological control agent for the Lucerne weevil, Sitona discoideus in native grassland Ferguson et al. 2016. The parasitoid also attacks New Zealand native weevil species both in pasture, and to a lesser extent in native grassland. The study sought to determine whether non-target parasitism in native grassland was a result of spill-over parasitism from lucerne areas near native grassland, or whether the parasitoid had become established in native weevil populations in the higher altitude natural grassland ecosystems (see figure).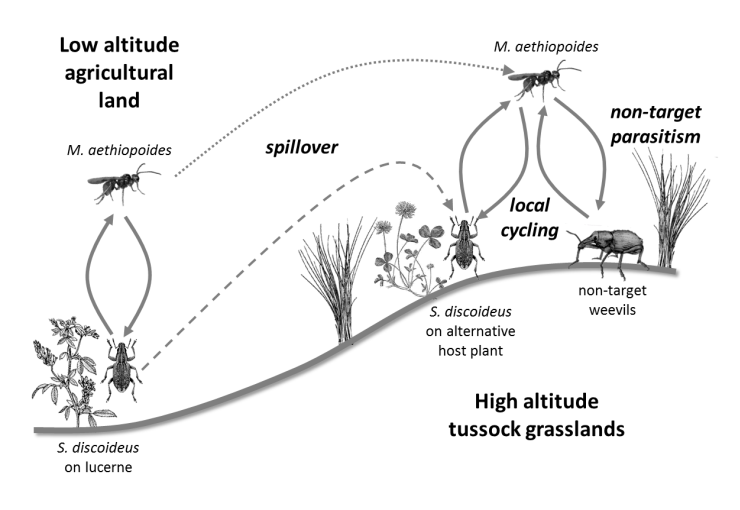
Schematic illustration of the processes hypothesized to lead to non-target parasitism of New Zealand native weevils by Microctonus aethiopoides in tussock grasslands.
© Copyright AgResearch, used with permission.
Indirect effects on other trophic levels and food webs
Specific recommendations in this area are extremely difficult, and studies
need to be designed on a case-by-case basis. In general, the ecology of the
system within which non-target parasitism is occurring needs to be very well
known if realistic indirect effects are to be measured. The ultimate goal of the
release of biological control agents is the restoration of invaded ecosystems.
Uninvaded reference sites or long-term documentation of communities before
release of biological control agents would provide useful benchmarks
(Blossey 1999). The current poor availability of biological inventories will make true
assessments of indirect impacts on food webs and species difficult. Monitoring
protocols need to be able to detect the extent to which the release of
biological control agents can drive population fluctuations or changes in
ecosystem function. Natural ecosystems are immensely complex, although invaded
systems may have lost a degree of their original complexity. However, the
prevalence of organism interactions makes it difficult to predict the response
of even well-understood systems to environmental change or perturbations
(Yodzis 1988, Polis and Strong 1996). Consequently, arguing with confidence that
conditions have ecologically improved or deteriorated, or are simply different
due to changes associated with spread of biological control agents, is
impossible unless such impacts can reliably be distinguished from natural
oscillations or plant succession. Lag effects make the detection and mitigation
of impacts even more challenging (Byers and Kendall 1982, Parker et al. 1999).
Laboratory and small-scale field experiments can not adequately replicate
interactions that occur in the field. The only way to capture the full range of
ecological effects of the release of biological control agents is by detailed
observations in actual ecosystems.
![]()
References
Barratt B.I.P., Blossey B. and Hokkanen H.M.T. (2006). Post-release evaluation of non-target effects of biological control agents. Pp. 166-186 In: Environmental Impact of Arthropod Biological Control: Methods and Risk Assessment, U. Kuhlmann, F. Bigler and D. Babendreier (Ed.) CABI Bioscience, Delemont, Switzerland.
Blossey B. (1999). Before, during and after: the need for long-term monitoring in invasive plant species management. Biological Invasions 1: 301-311.
Byers R.A. and Kendall W.A. (1982). Effects of plant genotypes and root nodulation on growth and survival of Sitona spp. larvae. Environmental Entomology 11: 440-443.
Ferguson C.M., Kean J.M., Barton D.M. and Barratt B.I.P. (2016). Ecological mechanisms for non-target parasitism by the Moroccan ecotype of Microctonus aethiopoides Loan (Hymenoptera: Braconidae) in native grassland. Biological Control 96: 28-38.
Holt R.D. and Hochberg M.E. (2001). Indirect interactions, community modules and biological control: a theoretical perspective. Pp. 13-37 In: Evaluating indirect ecological effects of biological control, E. Wajnberg, J. K. Scott and P. C. Quimby (Ed.) CABI Publishing, Wallingford, Oxon., UK
Hoogendorn M. and Heimpel G.E. (2003). PCR-Based gut content analysis of insect predators: A field study. Pp. 91-97 In: Proceedings of the 1st International Symposium on Biological Control of Arthropods, R. Van Driesche (Ed.) Forest Health Technology Enterprise Team, Morgantown, West Virginia.
Hopper K.R. (1998). Assessing and improving the safety of introductions for biological control. Pp. 501-510 In: Pest Management - Future Challenges: Proceedings of the 6th Australasian Applied Entomological Research Conference, M. Zalucki, R. Drew and G. White (Ed.) The Cooperative Research Centre for Tropical Pest Management.
Lynch L.D., Hokkanen H.M.T., Babendreier D., Bigler F., Burgio G., Gao Z.-H., Kuscke S., Loomans A., Menzler-Hokkanen I., Thomas M.B., Tommasini G., Waage J.K., Van Lenteren J.C. and Zeng Q.-Q. (2001). Insect biological control and non-target effects: a European perspective. Pp. 99-125 In: Evaluating indirect ecological effects of biological control, E. Wajnberg, J. K. Scott and P. C. Quimby (Ed.) CABI Publishing, Wallingford, Oxon., UK.
Parker I.M., Simberloff D., Lonsdale W.M., Goodell K., Wonham M., Kareiva P.M., Williamson M.H., Von Holle B., Moyle P.B., Byers J.E. and Goldwasser L. (1999). Impact: toward a framework for understanding the ecological effects of invaders. Biological Invasions 1: 3-19.
Polis G.A. and Strong D.R. (1996). Food web complexity and community dynamics. The American Naturalist 147: 813-846.
Stanley J.N. and Julien M.H. (1998). The need for post-release studies to improve risk assessments and decision making in classical biological control. Pp. 561-564 In: Pest Management - Future Challenges: Proceedings of the 6th Australasian Applied Entomological Research Conference, M. Zalucki, R. Drew and G. White (Ed.) The Cooperative Research Centre for Tropical Pest Management.
van Lenteren J.C., Cock M.J.W., Hoffmeister T.S. and Sands D.P.A. (2006). Host specificity in arthropod biological control, methods for testing and interpreting the data. Pp. 38-63. CAB Publishing, Delemont.
Yodzis P. (1988). The indeterminancy of ecological interactions as perceived through perturbation experiments. Ecology 69: 508-515.
Releasing biological control agents | Annotated bibliography |
|---|
